







The green fluorescent protein (GFP) is a protein composed of 238 amino acid residues (26.9 kDa) that exhibits bright green fluorescence when exposed to light in the blue to ultraviolet range.Although many other marine organisms have similar green fluorescent proteins, GFP traditionally refers to the protein first isolated from the jellyfish Aequorea victoria. The GFP from A. victoria has a major excitation peak at a wavelength of 395 nm and a minor one at 475 nm. Its emission peak is at 509 nm, which is in the lower green portion of the visible spectrum. The fluorescence quantum yield (QY) of GFP is 0.79. The GFP from the sea pansy (Renilla reniformis) has a single major excitation peak at 498 nm.
History
In the 1960s and 1970s, GFP, along with the separate luminescent protein aequorin (an enzyme that catalyzes the breakdown of luciferin, releasing light), was first purified from Aequorea victoria and its properties studied by Osamu Shimomura.In A. victoria, GFP fluorescence occurs when aequorin interacts with Ca2+ ions, inducing a blue glow. Some of this luminescent energy is transferred to the GFP, shifting the overall color towards green.However, its utility as a tool for molecular biologists did not begin to be realized until 1992 when Douglas Prasher reported the cloning and nucleotide sequence of wtGFP in Gene.The funding for this project had run out, so Prasher sent cDNA samples to several labs. The lab of Martin Chalfie expressed the coding sequence of wtGFP, with the first few amino acids deleted, in heterologous cells of E. coli and C. elegans, publishing the results in Science in 1994.Frederick Tsuji's lab independently reported the expression of the recombinant protein one month later.Remarkably, the GFP molecule folded and was fluorescent at room temperature, without the need for exogenous cofactors specific to the jellyfish. Although this near-wtGFP was fluorescent, it had several drawbacks, including dual peaked excitation spectra, pH sensitivity, chloride sensitivity, poor fluorescence quantum yield, poor photostability and poor folding at 37 °C.
The first reported crystal structure of a GFP was that of the S65T mutant by the Remington group in Science in 1996.One month later, the Phillips group independently reported the wild-type GFP structure in Nature Biotech.These crystal structures provided vital background on chromophore formation and neighboring residue interactions. Researchers have modified these residues by directed and random mutagenesis to produce the wide variety of GFP derivatives in use today. Martin Chalfie, Osamu Shimomura and Roger Y. Tsien share the 2008 Nobel Prize in Chemistry for their discovery and development of the green fluorescent protein.
Structure
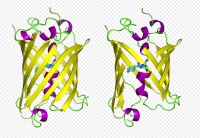
GFP has a beta barrel structure consisting of eleven ?-strands, with an alpha helix containing the covalently bonded chromophore 4-(p-hydroxybenzylidene)imidazolidin-5-one (HBI) running through the center. Five shorter alpha helices form caps on the ends of the structure. The beta barrel structure is a nearly perfect cylinder, 42Å long and 24Å in diameter,creating what is referred to as a “?-can” formation, which is unique to the GFP-like family.HBI, the spontaneously modified form of the tripeptide Ser65–Tyr66–Gly67, is nonfluorescent in the absence of the properly folded GFP scaffold and exists mainly in the un-ionized phenol form in wtGFP.Inward-facing sidechains of the barrel induce specific cyclization reactions in Ser65–Tyr66–Gly67 that induce ionization of HBI to the phenolate form and chromophore formation. This process of post-translational modification is referred to as maturation.The hydrogen-bonding network and electron-stacking interactions with these sidechains influence the color, intensity and photostability of GFP and its numerous derivatives.The tightly packed nature of the barrel excludes solvent molecules, protecting the chromophore fluorescence from quenching by water.